Corona medicine update: These 5 states were the first to get Corona medicine
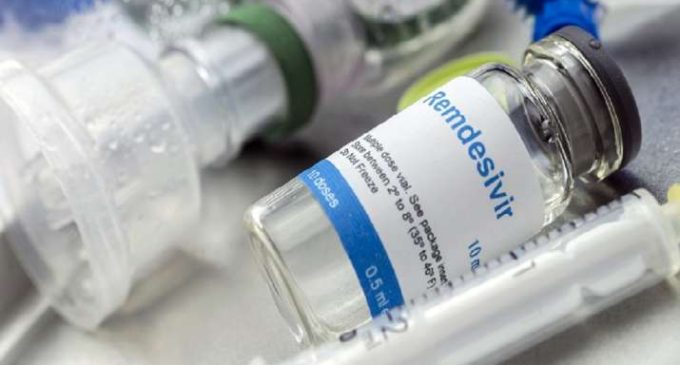
Covid-19 medicine news: 20,000 vials of a generic version of the drug Remdesivir used in the treatment of Kovid-19 have been sent to five states. This injection is named COVIFOR.
Highlights
- Kovifor is a generic version of the drug Remedesivir used to treat corona
- Hyderabad-based company Hetero prepared 20,000 injections in India
- Medicines dispatched to five states including Delhi, Maharashtra,
- The price of 100 mg vial was kept at Rs 5,400.
- Target to produce one lakh violations in three-four weeks
The generic drug of Corona virus has been sent to five states. Hedro, a bespoke company in Hyderabad, has created a generic version of Remdesvir under the name of Koviphor. The company has sent the first consignment of 20,000 vials to states like Delhi, Maharashtra, Gujarat, and Tamil Nadu which are badly affected by Corona. The first consignment of medicine will be used in Hyderabad, the capital of Telangana, where the company is located. According to Hetero, a 100 mg vial of coviphor will be available for Rs 5,400. The company has set a target to produce 100,000 lacs in the next three-four weeks.
The next consignment of medicine will be sent here
Currently, this injection is being made at the company’s formulation facility in Hyderabad. The drug’s active pharmaceutical ingredient (API) is being manufactured in a unit in Visakhapatnam. The next consignment of medicine will be sent to Bhopal, Indore, Kolkata, Patna, Lucknow, Ranchi, Bhubaneswar, Kochi, Vijayawada, Goa, and Trivandrum. At present, this medicine is available only through hospitals and the government, not at medical stores.
Another version of Remdesivir is ready
The pharmaceutical company Cipla has signed a licensing agreement with American company Gilead Sciences Inc, which manufactures Remedisvir. Cipla will also make and sell this medicine. The company says that its medicine will be available for less than Rs 5,000. The Drug Controller General of India (DGCI), the regulator of allopathic medicines in India, has given permission to both Cipla and Hetero to manufacture and sell medicines for restricted emergency use on severe COVID-19 patients.
Generic version of Favipiravir also exists
DGCI has also previously approved Glenmark Pharmaceuticals to make a generic version of the antiviral drug Favipiravir. The company manufactures this drug under the name FabiFlu. A full strip of 34 tablets will be available for Rs 3,500 i.e. a tablet will cost around Rs 103. The drug FabiFlu will be used to treat patients with mild to moderate symptoms. It will be found in drug hospitals and medical stores on prescription.
Remadecivir reduces recovery time
The results of Remdesvir in the trial were good. According to The New England Journal of Medicine (NEJM), Remdesivir’s 10-day course proved more effective than a placebo in the treatment of Kovid-19 patients. The time taken for recovery after treatment in both methods was shorter in Remdesivir. Researchers suggested that this drug may be used on patients who are suffering from Kovid-19 and who need supplemental oxygen therapy.






There are no comments at the moment, do you want to add one?
Write a comment